Your contamination control partner
Cleanroom contamination control solutions are essential for any company performing processes under cleanroom conditions. After carrying out a needs assessment, we offer you the best cleanroom textiles and service solutions suitable for your specific controlled environment and required classifications.
As an Elis customer, we ensure that you always have sufficient resources and that they comply with the latest, stringent standards, recommendations and guidelines. You have nothing to worry about. We take care of purchasing, decontaminating, sterilising, repairing, replacing and managing your clothing and/or cleaning products.
With decades of experience in the cleanroom industry, we provide the best options for your controlled environment. We will identify your needs, evaluate your SOP and advise you on the use of the right cleanroom outer garments, inner garments, accessories and appliances.

All this to protect your products and production from external and human contamination. In doing so, we relieve you of all your worries. We arrange a tailored fit rental solution, from stock, sterilisation, delivery, maintenance and management to replacement
Why choose Elis Cleanroom?
Guaranteed quality
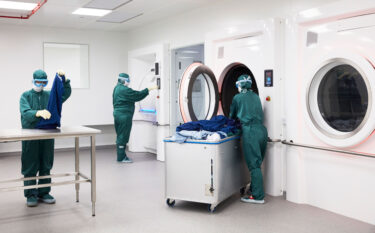
In our specialised cleanroom laundries, the risk of cross-contamination is eliminated not only through continuous monitoring, but also by performing on predefined testing and controls and internal and external audits. In our laundries, we have one or more certified cleanrooms: ISO Class 5 (Grade A/B) and ISO Class 7 (Grade C/D).
Full service
We offer you flexibility and advice, not only on the type of garments but also on how to use them. Through the online management portal Elis Connect, you can see and follow the cycle and usage of your clothing and textiles.
Security of delivery
We hold and manage our own vehicle fleet with trained permanent drivers, a back-up plan in case of emergencies in our own laundry, including a contingency option in at least one of our 33 cleanroom decontamination units.
Qualified documentation
We work according to GMP, documenting everything we do and acting in accordance with: ISO 9001:2008 (Quality Management System), ISO 14001 (Environment), ISO 14644 (Low-dust and low-germ rooms and environments), ISO 17655 (Sterilisation of medical devices), IEST RP-CC003.5 and ISO 14698





















